-
+971 55 703 4562
+971 55 703 4562
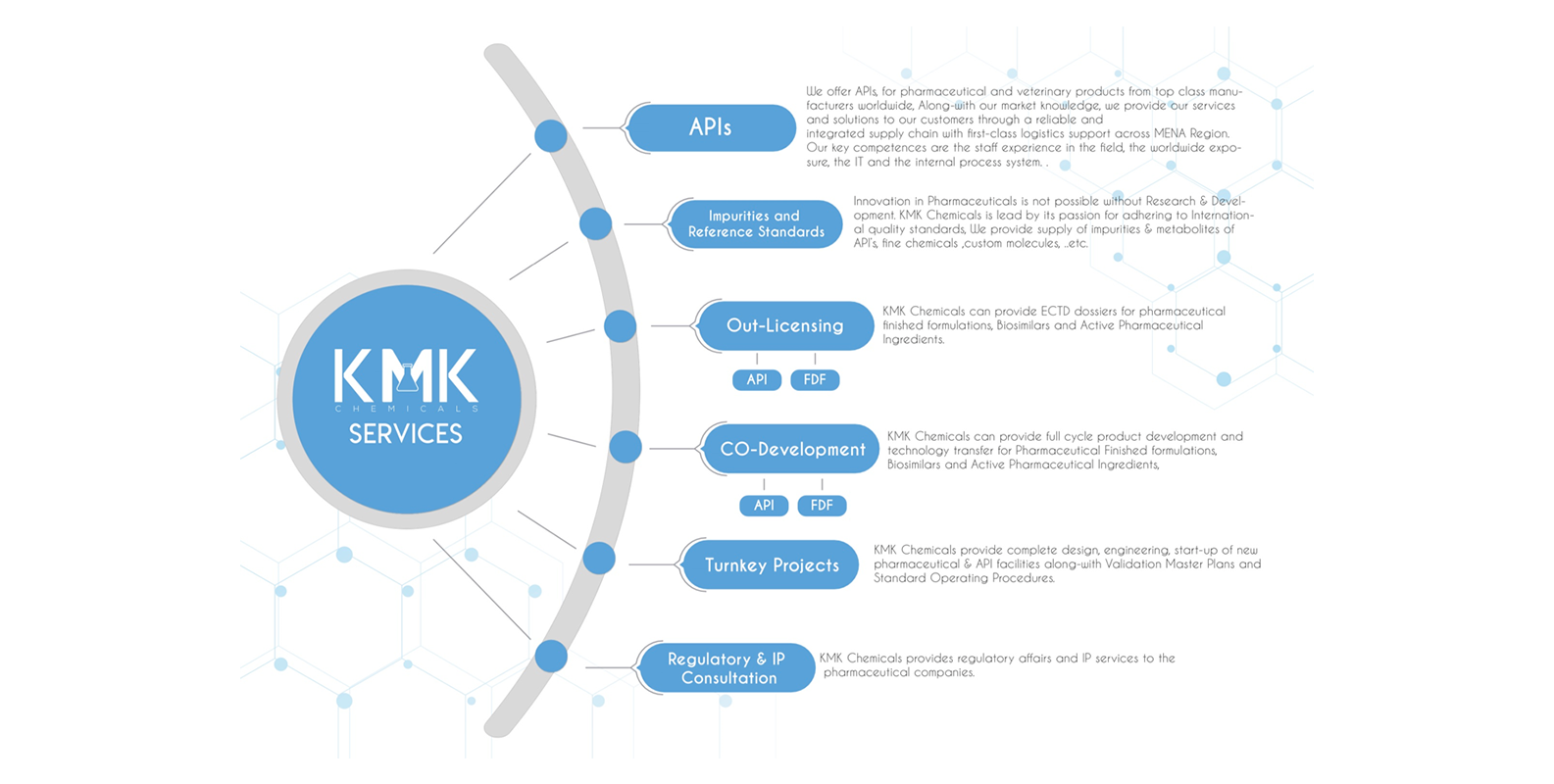
KMK CHEMICALS is a leading distributor and an ISO certified company specialized in sourcing and distributing Active Pharmaceutical Ingredients and Finished Formulations in MENA region, Being a customer-oriented company, we continuously enlarge our portfolio to be able to offer smart solutions and to meet most of the requirements of our customers in an easy way. Our team always share their technical and business insights to our customers for their new and existing products. Knowing the importance of our suppliers as well, KMK uses advanced ways to facilitate business transactions with them. We developed our digital channel (KMK Mobile Application) to be able to serve our wide-ranging networks of suppliers and customers in a smart way.

Excellence and prevalence in submitting company and customer service’s considering our code of ethics. Excellence and superiority in professional development and in applying information technology with the use of capital manpower. Excellence and efficiency in developing the raw materials industry and in health activities participation that serves the community in the MENA region.

KMK’s vision is to empower Pharmaceutical companies by being one of the top five distributors of Active Pharmaceutical Ingredients and Pharmaceutical Finished formulations in the MENA region . Our objective is to be perceived as the first choice for our customers when it comes to API & FDF sourcing.

- Excellence
- Performance
- Integrity
- Accountability
- Market Oriented